May 2023
Rafal Nowak, MD, PhD Department of Ophthalmology, Military Institute of Medicine, Warsaw, Poland
Correspondence to: drrafal007@gmail.com Aim To present successful outcome of endoscopic endonasal dacryocystorhinostomy (EnDCR) in posttraumatic lacrimal drainage obstruction caused by the Le Fort fractures. Introduction Dacryocystorhinostomy (DCR) refers to the creation of a functional pathway for tears to drain into the nasal cavity by means of making an osteotomy and opening the lacrimal sac directly into the nasal cavity1. This kind of surgery is indicated in cases of nasolacrimal duct obstruction (NLDO), which can further cause chronic dacryocystitis with acute episodes2 (Fig.1). The procedure can be performed via an external or endoscopic endonasal approach. Some surgeons conduct laser-assisted transcanalicular DCR, although it does not provide good results3. DCR in primary acquired NLDO is a standard treatment, however, in posttraumatic cases it may turn out challenging.
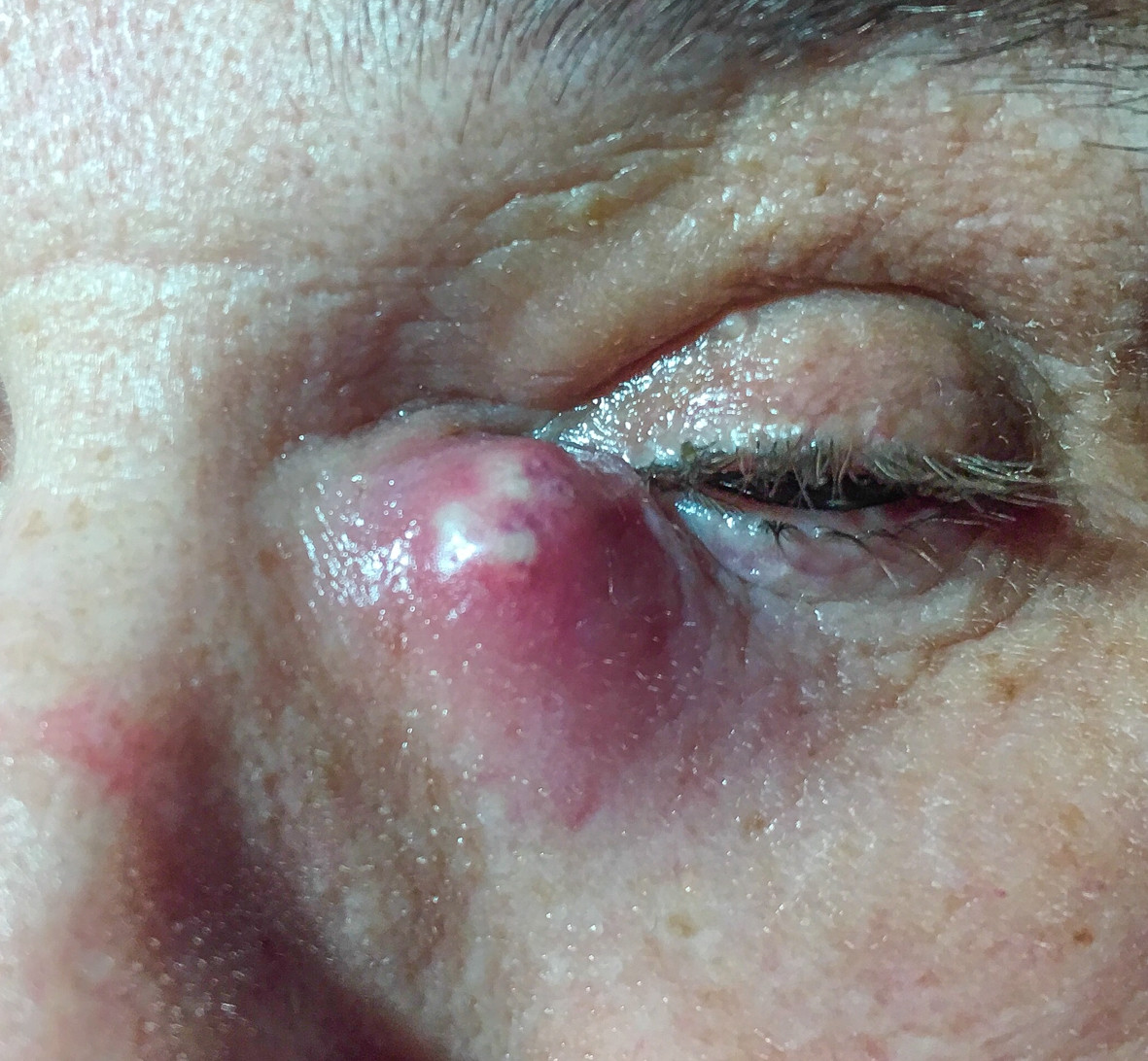 Figure 1. Acute dacryocystitis.
Secondary posttraumatic NLDO usually results from a high-impact blunt
facial trauma causing naso-orbito-ethmoidal (NOE) fractures, often being
a part of the Le Fort fractures4, 5 (Fig.2-3). Since the
upper lacrimal pathway is protected by the medial canthal ligament,
lacrimal obstruction usually occurs in the bony nasolacrimal canal.
Consequently, the anatomy of the lacrimal fossa and nasolacrimal duct
(NLD) is altered with a lot of scarring, and the surgery carries an
increased risk of failure. Some studies prefer external DCR or
dacryocystectomy (DCT; removal of the lacrimal sac) in complex
posttraumatic cases of NLDO6. They discourage endoscopic
endonasal or laser DCR due to the loss of bony anatomical landmarks
after trauma7.
Figure 1. Acute dacryocystitis.
Secondary posttraumatic NLDO usually results from a high-impact blunt
facial trauma causing naso-orbito-ethmoidal (NOE) fractures, often being
a part of the Le Fort fractures4, 5 (Fig.2-3). Since the
upper lacrimal pathway is protected by the medial canthal ligament,
lacrimal obstruction usually occurs in the bony nasolacrimal canal.
Consequently, the anatomy of the lacrimal fossa and nasolacrimal duct
(NLD) is altered with a lot of scarring, and the surgery carries an
increased risk of failure. Some studies prefer external DCR or
dacryocystectomy (DCT; removal of the lacrimal sac) in complex
posttraumatic cases of NLDO6. They discourage endoscopic
endonasal or laser DCR due to the loss of bony anatomical landmarks
after trauma7.
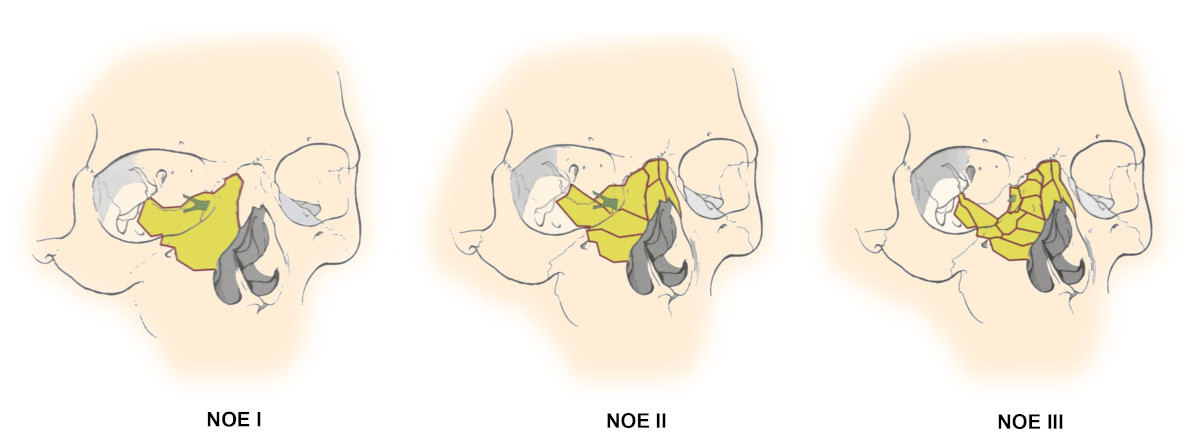 Figure 2. Types of NOE fractures8.
Figure 2. Types of NOE fractures8.
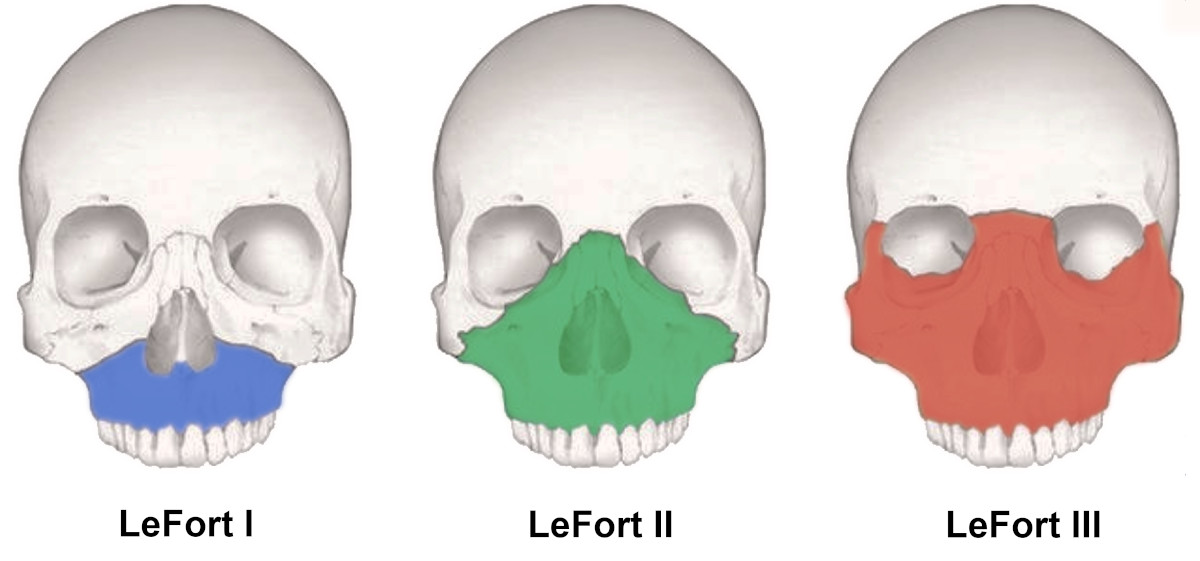 Figure 3. Types of Le Fort fractures9.
Materials and methods
This study refers to 3 cases of secondary posttraumatic nasolacrimal
duct obstruction following Le Fort fractures. The surgeries of EnDCR
were performed at the Department of Ophthalmology of the Military
Institute of Medicine in Warsaw within the period of 1.5 years: November
2020 - May 2022. The follow-up time was 1-2.5 years.
Apart from standard dacryological examination, extended imaging of
computed tomography-dacryocystography (CT-DCG) was conducted. The images
were analyzed in Osirix MD radiological software (Pixmeo SARL,
Switzerland). For further evaluation and surgical planning
Medical
Imaging XR
(Medicalholodeck AG, Switzerland) was used. Then, 3D reconstructions
were made using the following processes:
STL* 3D model extraction from OsiriX MD STL* model cleaning, repairing,
simplification and modification with MeshLab (Visual Computing Lab,
ISTI-CNR, Italy) final processing in Blender (Blender Foundation,
Netherlands) and obtaining color 3D models *STL - 3D file format
After careful analysis of all collected data, the surgeries were
performed (Fig. 4-11).
Figure 3. Types of Le Fort fractures9.
Materials and methods
This study refers to 3 cases of secondary posttraumatic nasolacrimal
duct obstruction following Le Fort fractures. The surgeries of EnDCR
were performed at the Department of Ophthalmology of the Military
Institute of Medicine in Warsaw within the period of 1.5 years: November
2020 - May 2022. The follow-up time was 1-2.5 years.
Apart from standard dacryological examination, extended imaging of
computed tomography-dacryocystography (CT-DCG) was conducted. The images
were analyzed in Osirix MD radiological software (Pixmeo SARL,
Switzerland). For further evaluation and surgical planning
Medical
Imaging XR
(Medicalholodeck AG, Switzerland) was used. Then, 3D reconstructions
were made using the following processes:
STL* 3D model extraction from OsiriX MD STL* model cleaning, repairing,
simplification and modification with MeshLab (Visual Computing Lab,
ISTI-CNR, Italy) final processing in Blender (Blender Foundation,
Netherlands) and obtaining color 3D models *STL - 3D file format
After careful analysis of all collected data, the surgeries were
performed (Fig. 4-11).
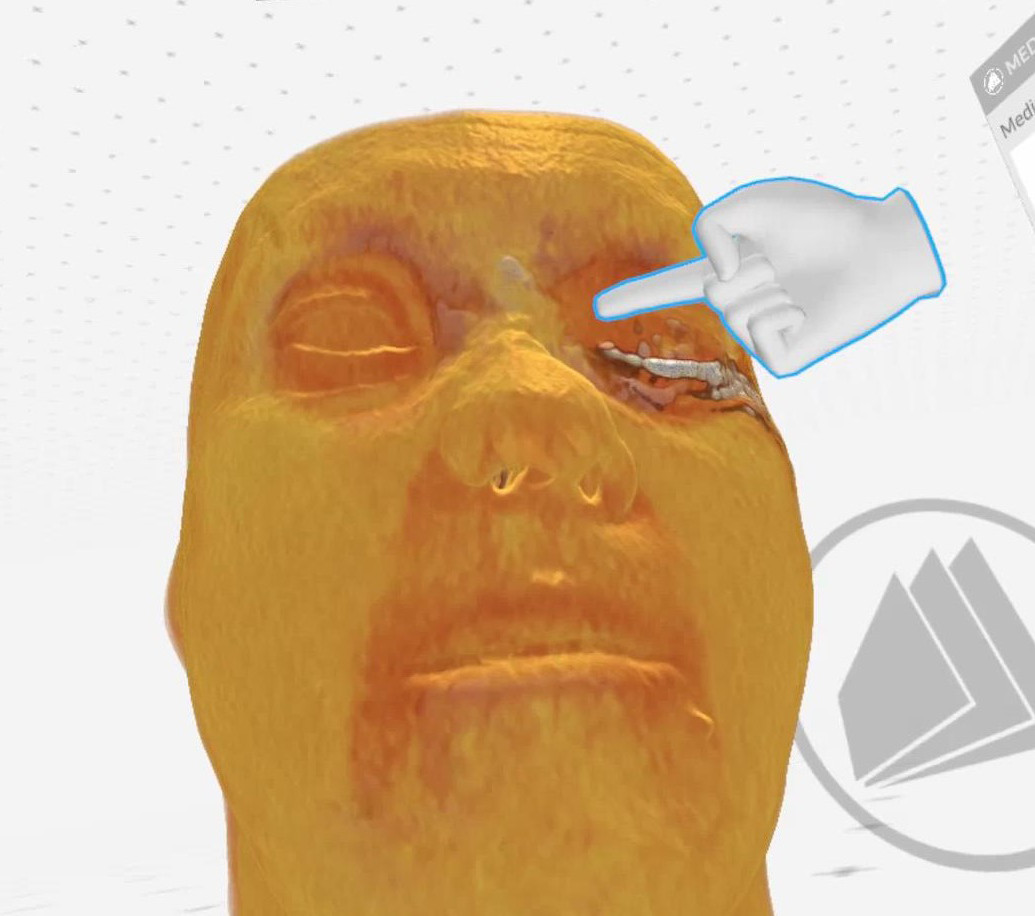 Figure 4. Case 1 (left side): Saddle nose (Medical Imaging XR
reconstruction).
Figure 4. Case 1 (left side): Saddle nose (Medical Imaging XR
reconstruction).
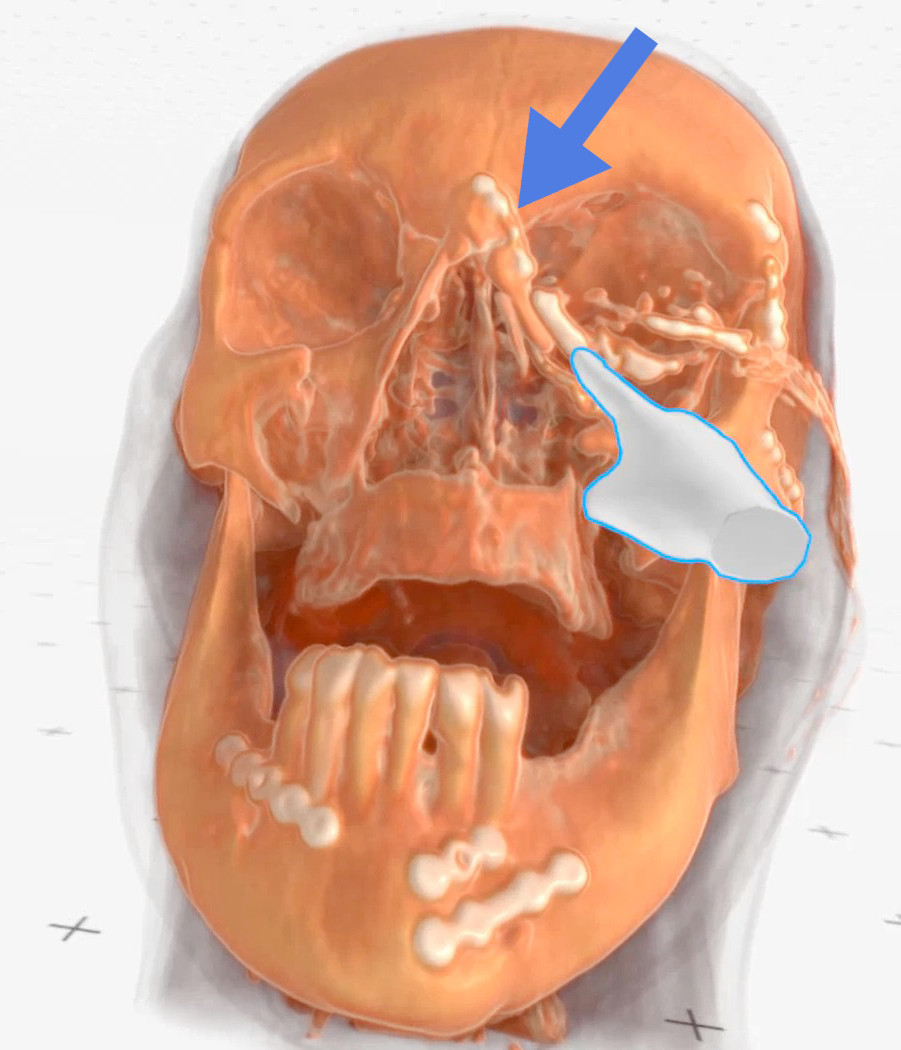 Figure 5. Case 1 (left side): Finger points at the lacrimal sac, blue
arrow - indicates a metal plate neighboring the lacrimal sac (Medical
Imaging XR reconstruction).
Figure 5. Case 1 (left side): Finger points at the lacrimal sac, blue
arrow - indicates a metal plate neighboring the lacrimal sac (Medical
Imaging XR reconstruction).
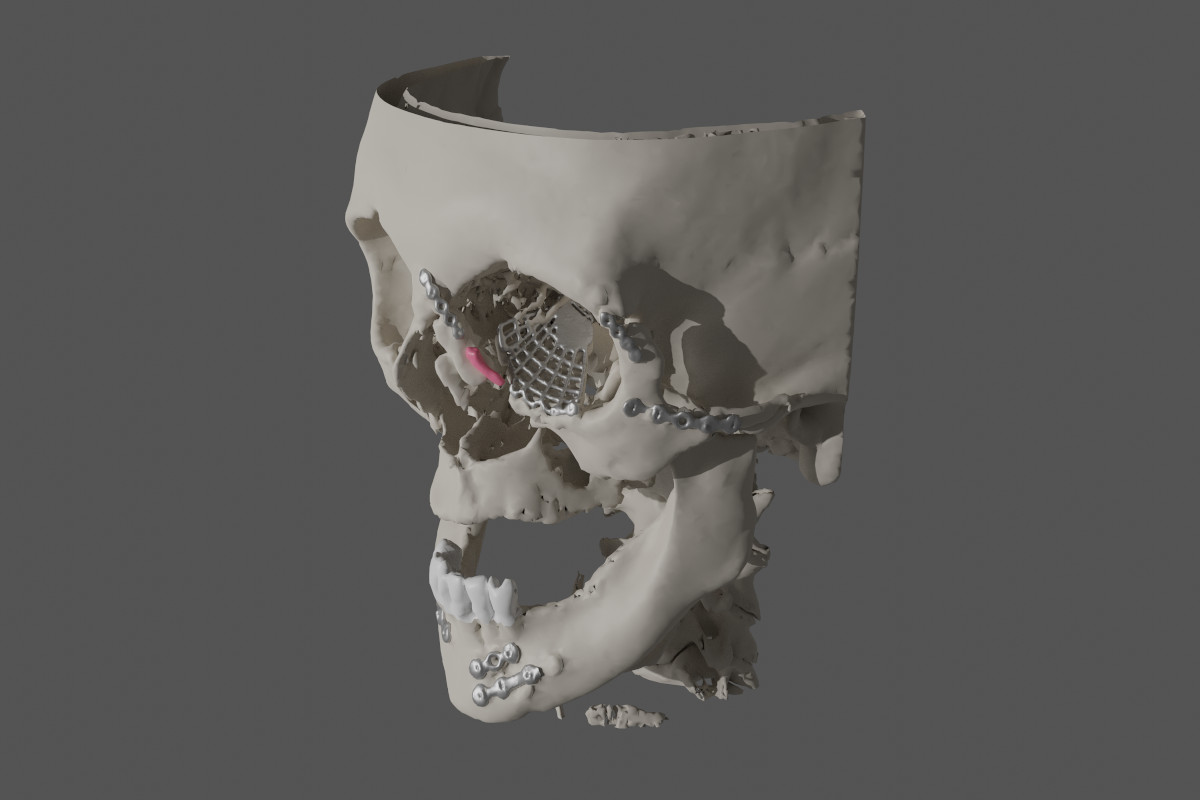 Figure 6. Case 1 (left side): Numerous metal plates and an orbital mesh;
lacrimal sac marked in pink (reconstruction made with OsiriX
MD/MeshLab/Blender).
Figure 6. Case 1 (left side): Numerous metal plates and an orbital mesh;
lacrimal sac marked in pink (reconstruction made with OsiriX
MD/MeshLab/Blender).
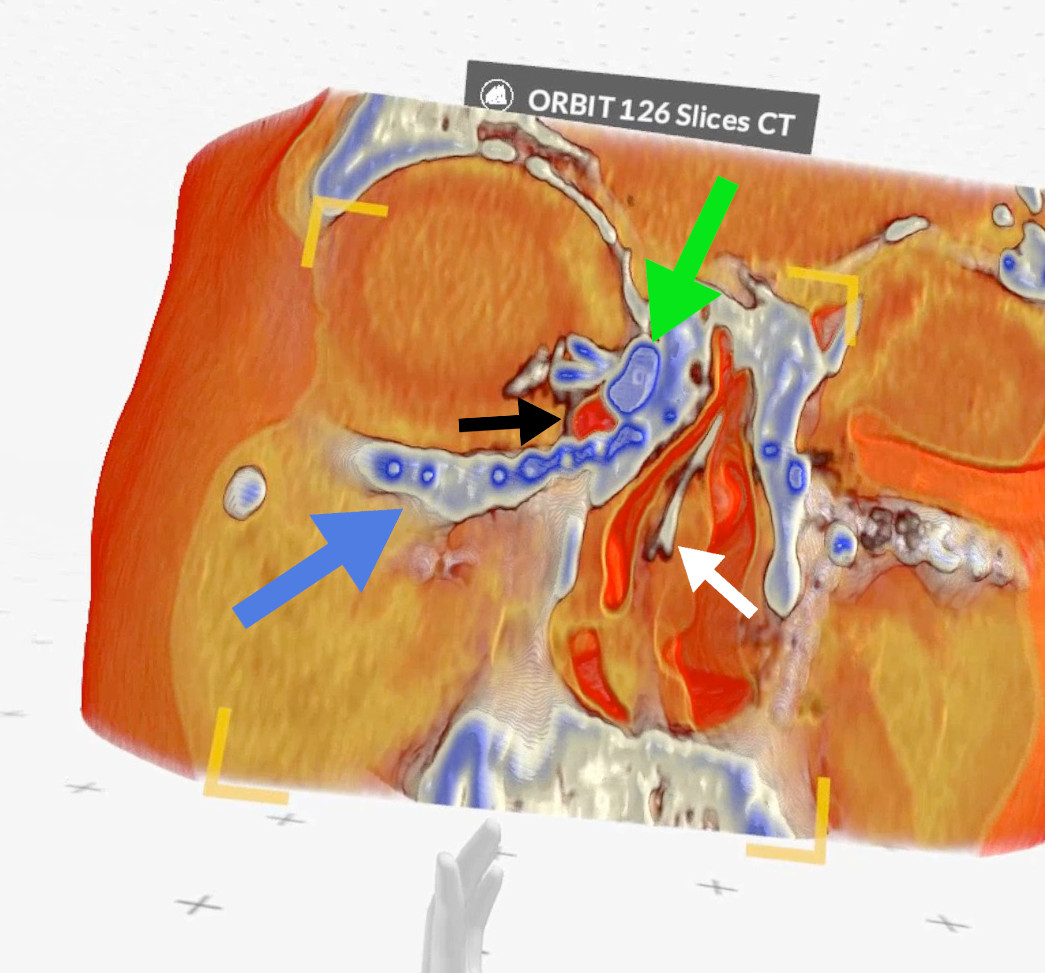 Figure 7. Case 2 (right side): green arrow - lacrimal sac, black arrow -
air bubble inside the lacrimal sac, blue arrow - metal plate, white
arrow - septal deviation (Medical Imaging XR reconstruction).
Figure 7. Case 2 (right side): green arrow - lacrimal sac, black arrow -
air bubble inside the lacrimal sac, blue arrow - metal plate, white
arrow - septal deviation (Medical Imaging XR reconstruction).
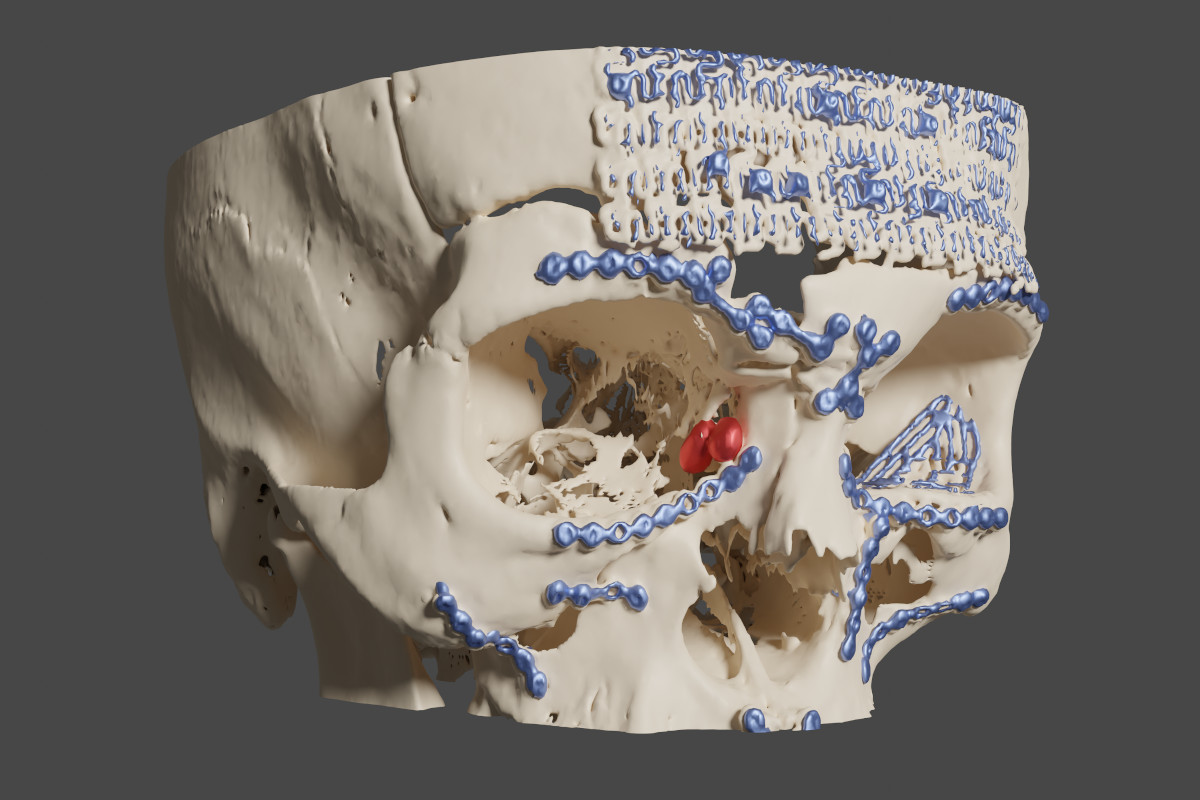 Figure 8. Case 2 (right side): blue - metal plates and meshes, red -
lacrimal sac (reconstruction made with OsiriX MD/MeshLab/Blender).
Figure 8. Case 2 (right side): blue - metal plates and meshes, red -
lacrimal sac (reconstruction made with OsiriX MD/MeshLab/Blender).
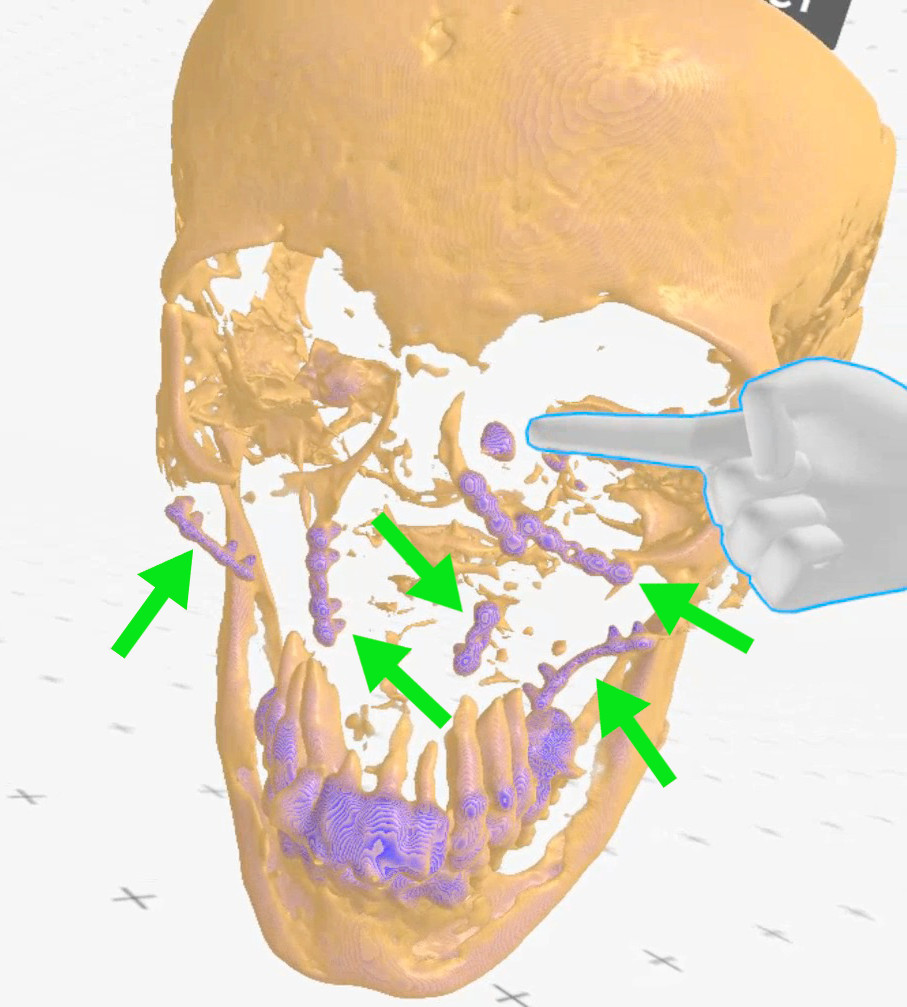 Figure 9. Case 3 (left side): finger points at the lacrimal sac, green
arrows - metal plates (Medical Imaging XR reconstruction).
Figure 9. Case 3 (left side): finger points at the lacrimal sac, green
arrows - metal plates (Medical Imaging XR reconstruction).
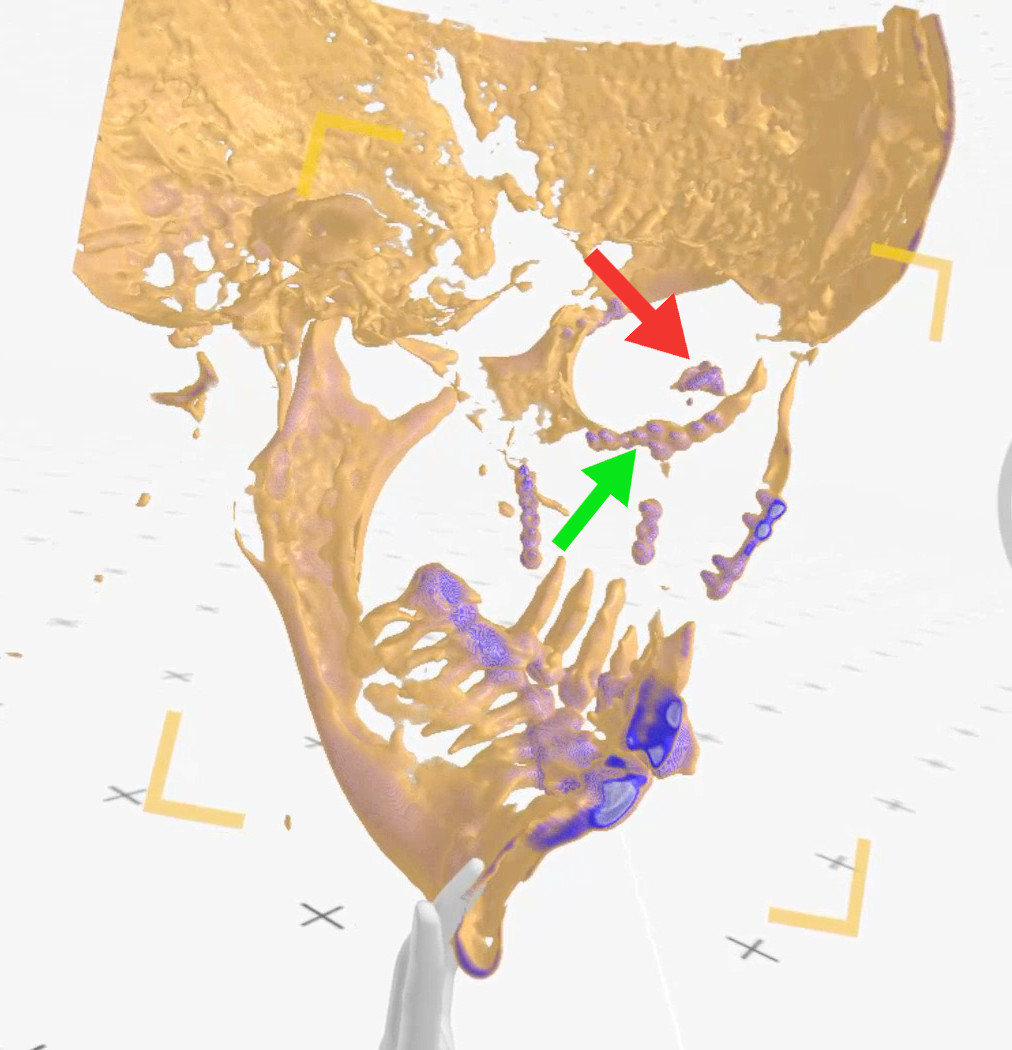 Figure 10. Case 3 (left side): view from inside the skull (from right to
left), red arrow points at the lacrimal sac, green arrow - indicates
metal plates neighboring the lacrimal sac (Medical Imaging XR
reconstruction).
Figure 10. Case 3 (left side): view from inside the skull (from right to
left), red arrow points at the lacrimal sac, green arrow - indicates
metal plates neighboring the lacrimal sac (Medical Imaging XR
reconstruction).
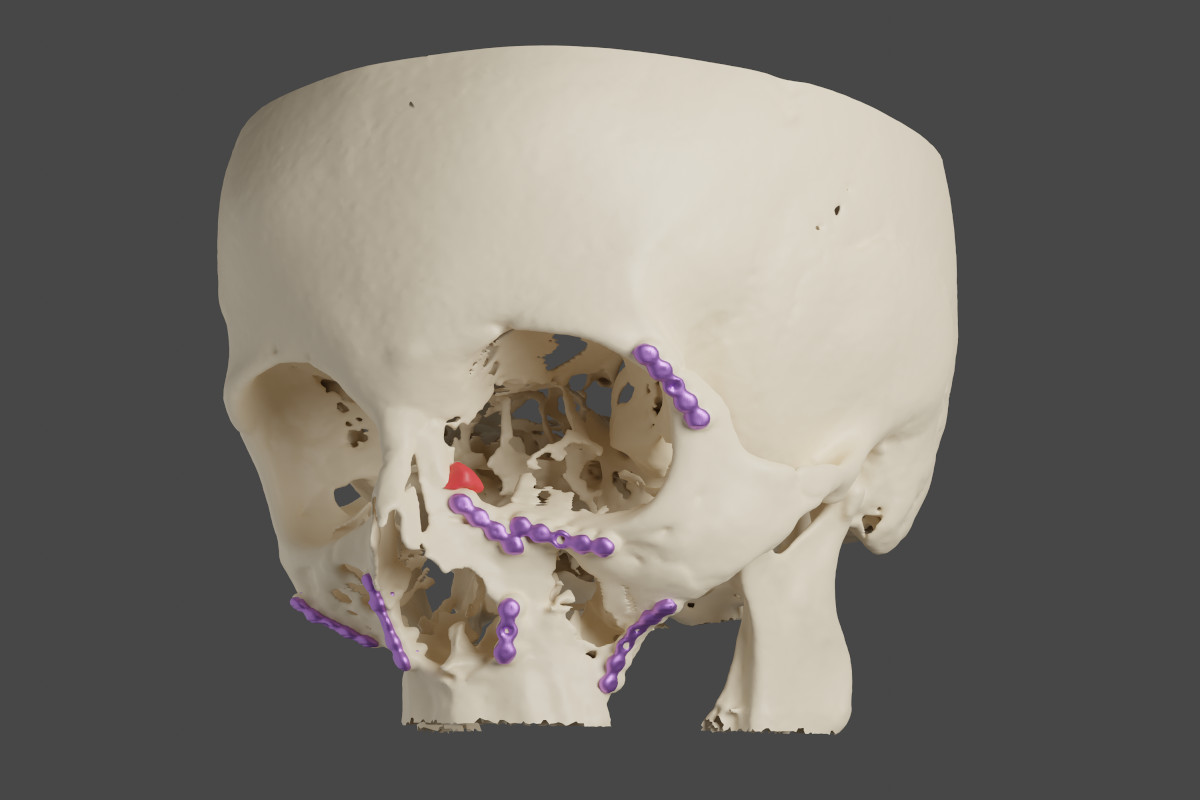 Figure 11. Case 3 (left side): red - the lacrimal sac, violet - metal
plates (reconstruction made with OsiriX MD/MeshLab/Blender).
Surgical procedure of EnDCR The surgical procedure was conducted as
described elsewhere10, 11, however, there were some
derogations in each operated case. In all of them, light navigation was
used to find the location of the lacrimal sac. Cases 1 and 2 had
posttraumatic saddle noses and septal deviations.
CASE 1
The procedure was performed on the left side. The lacrimal sac was
found under a thick layer of scar tissue, around 15mm anteriorly to the
base of the middle turbinate. Thanks to the previous 3D visualization,
it was known that a rim of a metal orbital mesh was located close to the
middle turbinate (Fig. 12). It came to the surface during dissection but
was then covered with a mucosal flap and tissue glue.
Figure 11. Case 3 (left side): red - the lacrimal sac, violet - metal
plates (reconstruction made with OsiriX MD/MeshLab/Blender).
Surgical procedure of EnDCR The surgical procedure was conducted as
described elsewhere10, 11, however, there were some
derogations in each operated case. In all of them, light navigation was
used to find the location of the lacrimal sac. Cases 1 and 2 had
posttraumatic saddle noses and septal deviations.
CASE 1
The procedure was performed on the left side. The lacrimal sac was
found under a thick layer of scar tissue, around 15mm anteriorly to the
base of the middle turbinate. Thanks to the previous 3D visualization,
it was known that a rim of a metal orbital mesh was located close to the
middle turbinate (Fig. 12). It came to the surface during dissection but
was then covered with a mucosal flap and tissue glue.
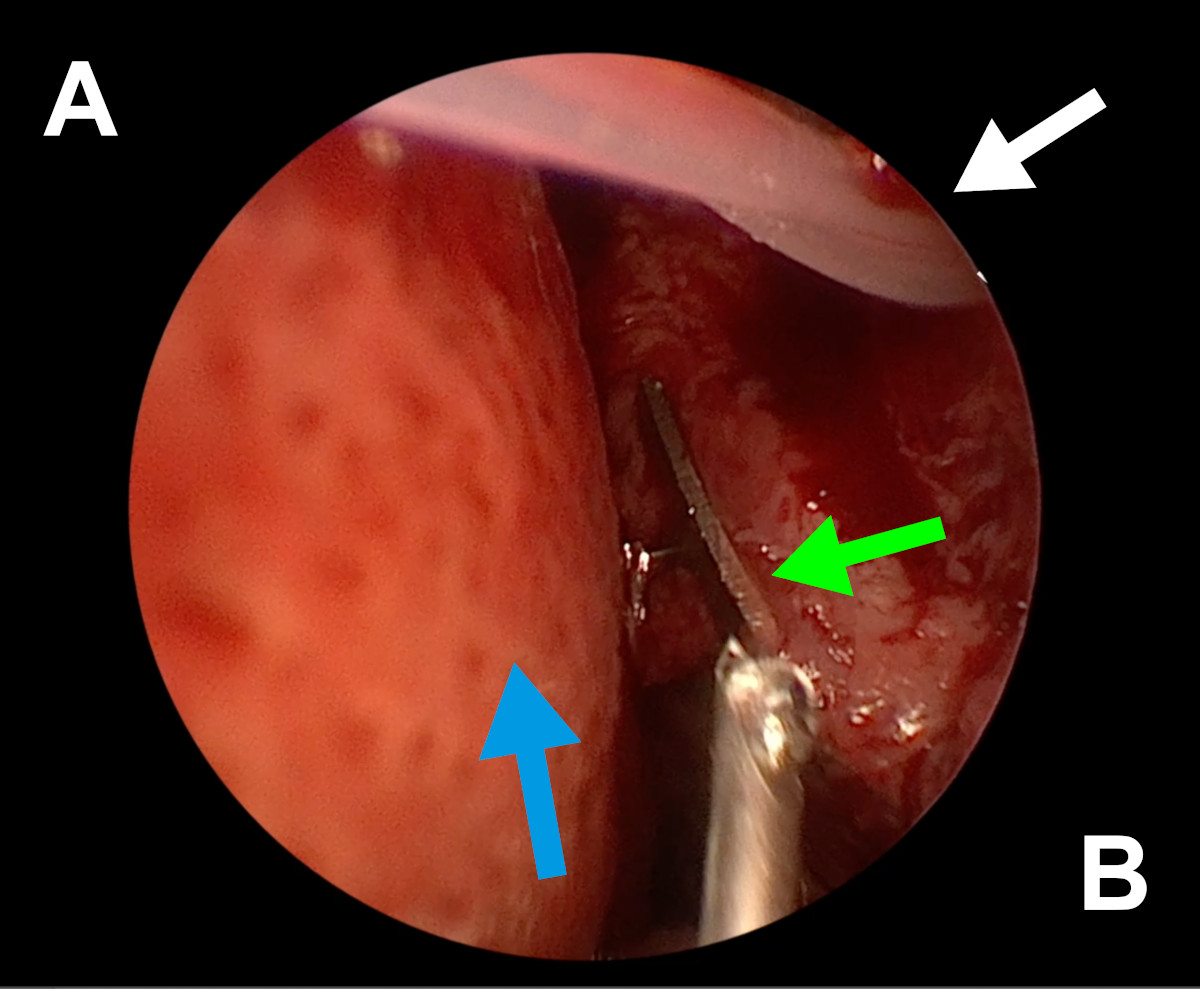 Figure 12. Endoscopic view of the left nasal cavity: white arrow – a
silicone stent coming out from ostium, green arrow – orbital mesh, blue
arrow – septal deviation (A- septum, B- lateral wall of the nasal
cavity).
CASE 2
The procedure was performed on the right side. While fashioning the
osteotomy, a tip of a metal bone screw was uncovered on its anterior
edge. It had been expected, so burring was conducted around it in such a
way that the neighboring nasal mucosa was able to cover it (Fig. 13-15).
Figure 12. Endoscopic view of the left nasal cavity: white arrow – a
silicone stent coming out from ostium, green arrow – orbital mesh, blue
arrow – septal deviation (A- septum, B- lateral wall of the nasal
cavity).
CASE 2
The procedure was performed on the right side. While fashioning the
osteotomy, a tip of a metal bone screw was uncovered on its anterior
edge. It had been expected, so burring was conducted around it in such a
way that the neighboring nasal mucosa was able to cover it (Fig. 13-15).
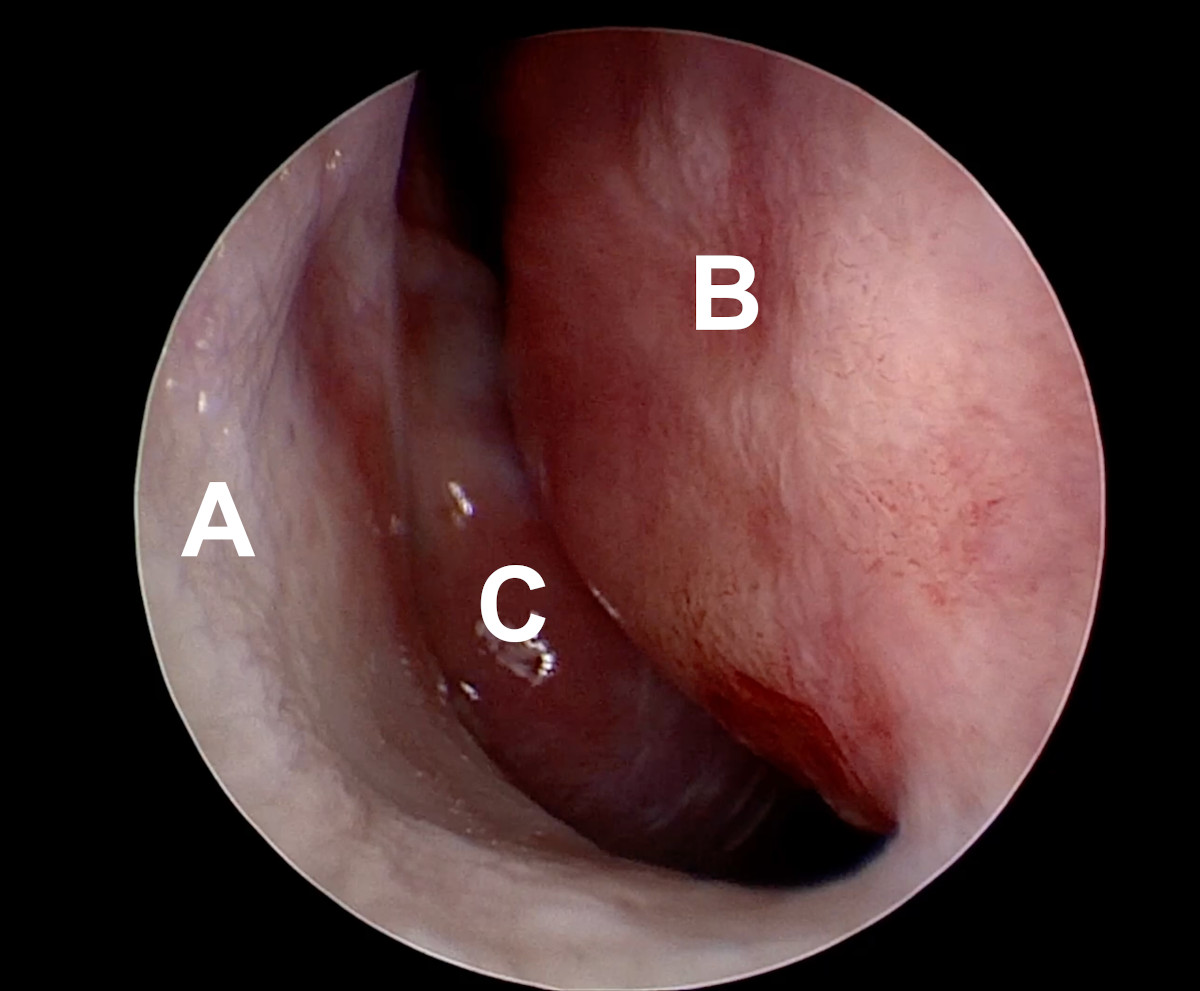 Figure 13. Endoscopic view of the right nasal cavity: septal deviation
(A- lateral wall of the nasal cavity, D- deviated nasal septum, C-
middle turbinate).
Figure 13. Endoscopic view of the right nasal cavity: septal deviation
(A- lateral wall of the nasal cavity, D- deviated nasal septum, C-
middle turbinate).
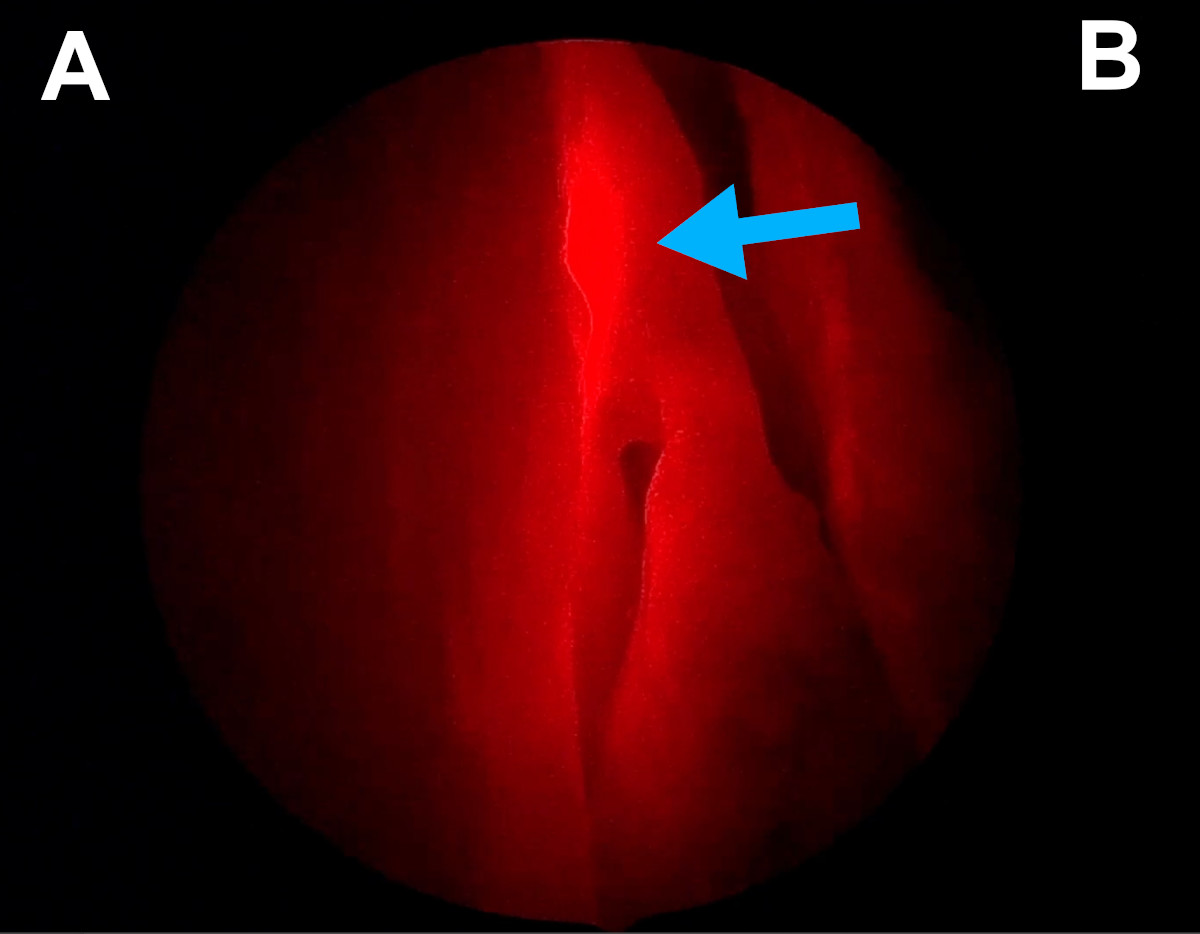 Figure 14. Endoscopic view of the right nasal cavity: light navigation
(blue arrow points at the red spot of a light probe introduced into the
lacrimal sac through the lacrimal canaliculus; the spot is located at
the base of the middle turbinate) [A- lateral wall of the nasal cavity,
B- septum].
Figure 14. Endoscopic view of the right nasal cavity: light navigation
(blue arrow points at the red spot of a light probe introduced into the
lacrimal sac through the lacrimal canaliculus; the spot is located at
the base of the middle turbinate) [A- lateral wall of the nasal cavity,
B- septum].
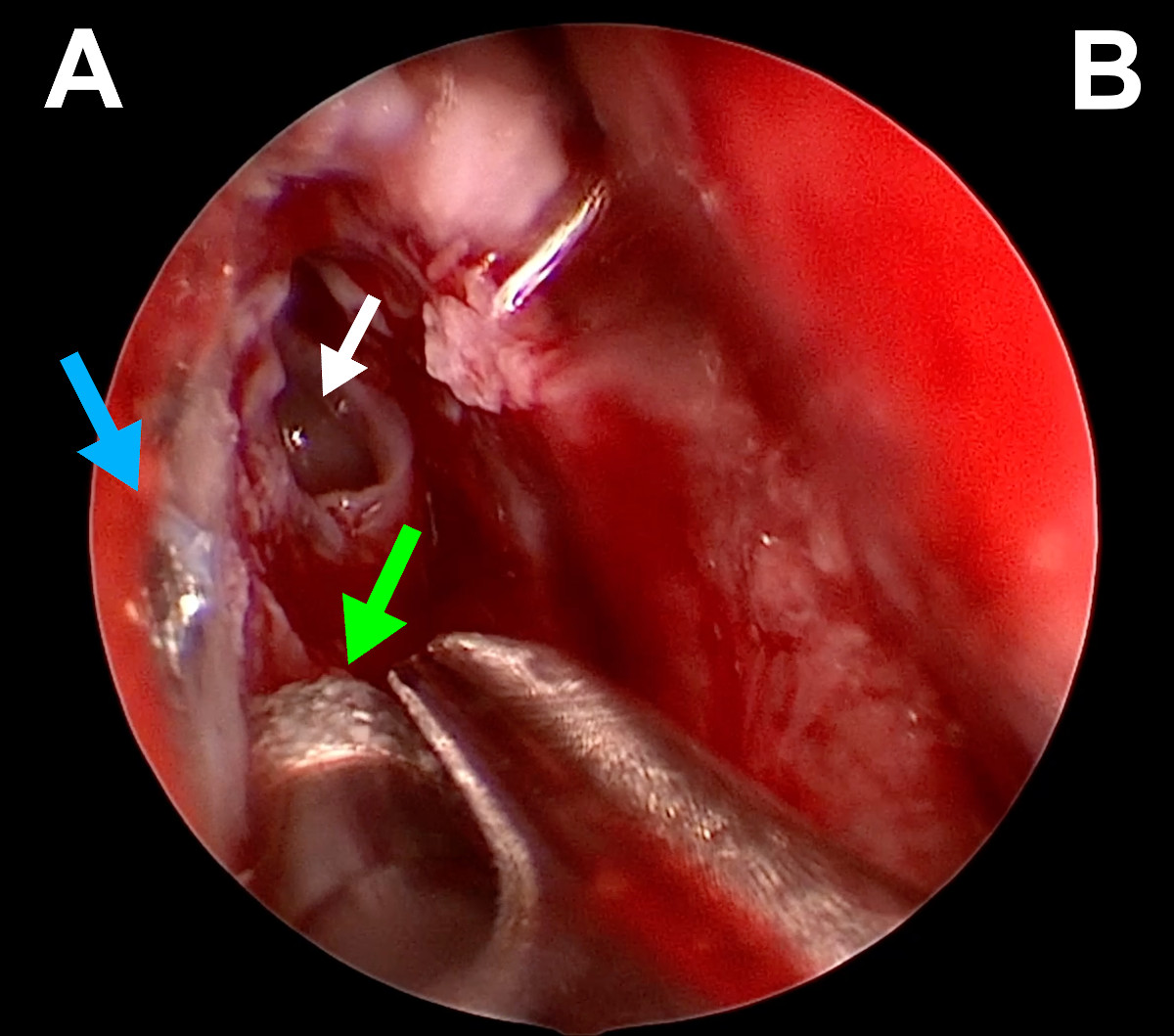 Figure 15. Endoscopic view of the right nasal cavity: green arrow –
diamond burr, white arrow – opened agger nasi, blue arrow – tip of a
metal bone screw (A- lateral wall of the nasal cavity, B- septum).
CASE 3
The procedure was performed on the left side. The lacrimal sac was found
under a thick layer of scar tissue, around 15mm anteriorly to the base
of the middle turbinate. Thanks to the previous 3D visualization, it was
known that a rim of a metal orbital mesh was located close to the middle
turbinate (Fig. 12). It came to the surface during dissection but was
then covered with a mucosal flap and tissue glue.
Figure 15. Endoscopic view of the right nasal cavity: green arrow –
diamond burr, white arrow – opened agger nasi, blue arrow – tip of a
metal bone screw (A- lateral wall of the nasal cavity, B- septum).
CASE 3
The procedure was performed on the left side. The lacrimal sac was found
under a thick layer of scar tissue, around 15mm anteriorly to the base
of the middle turbinate. Thanks to the previous 3D visualization, it was
known that a rim of a metal orbital mesh was located close to the middle
turbinate (Fig. 12). It came to the surface during dissection but was
then covered with a mucosal flap and tissue glue.
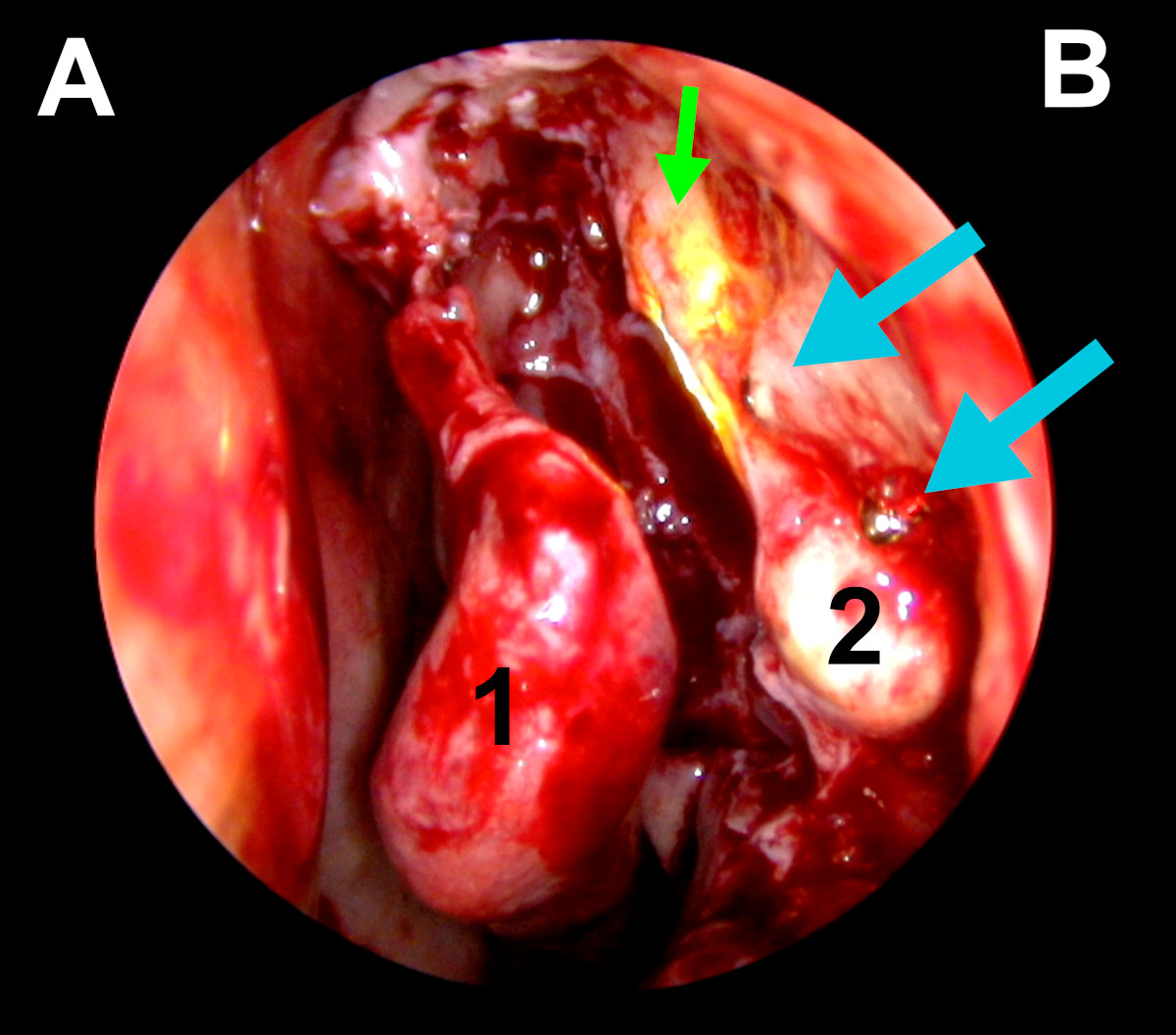 Figure 16. Endoscopic view of the left nasal cavity: blue arrows point
on tips of metal bone screws, green arrow- light navigation, 1-middle
turbinate, 2- exposed lacrimal sac, (A- septum, B- lateral wall of the
nasal cavity).
In all cases proper marsupialization of the lacrimal sac was performed.
The lacrimal pathway was intubated with a silicone stent. At the
completion of surgery, topical application and circumostial Mitomycin C
injection were conducted.
Results
In all of the described cases, lacrimal drainage system remained patent
within the follow-up time. Epiphora was absent (Fig. 17-18).
Figure 16. Endoscopic view of the left nasal cavity: blue arrows point
on tips of metal bone screws, green arrow- light navigation, 1-middle
turbinate, 2- exposed lacrimal sac, (A- septum, B- lateral wall of the
nasal cavity).
In all cases proper marsupialization of the lacrimal sac was performed.
The lacrimal pathway was intubated with a silicone stent. At the
completion of surgery, topical application and circumostial Mitomycin C
injection were conducted.
Results
In all of the described cases, lacrimal drainage system remained patent
within the follow-up time. Epiphora was absent (Fig. 17-18).
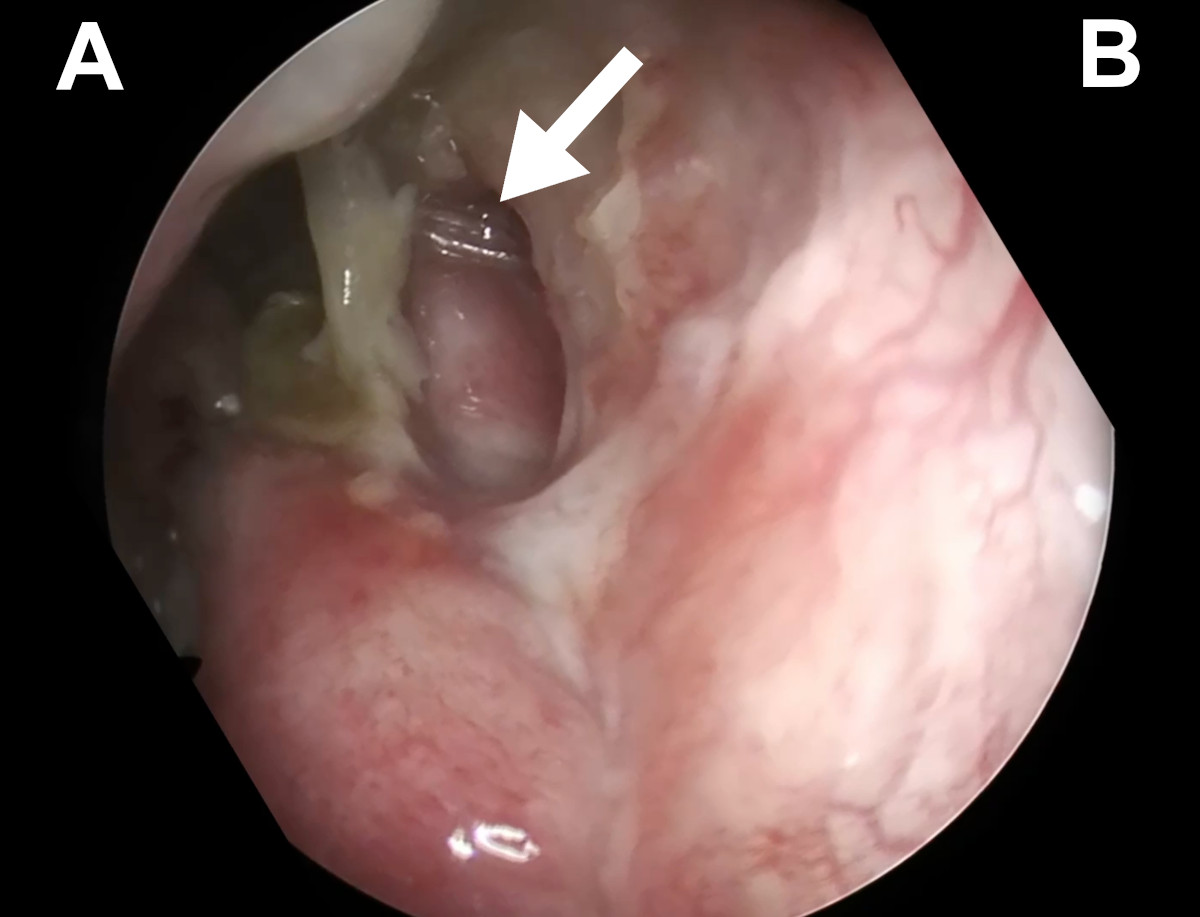 Figure 17. Endoscopic view of the left nasal cavity at 6 weeks postop
(Case 1): white arrow points at the silicone stent coming out from a
patent ostium (A- nasal septum, B- lateral wall of the nasal cavity).
Figure 17. Endoscopic view of the left nasal cavity at 6 weeks postop
(Case 1): white arrow points at the silicone stent coming out from a
patent ostium (A- nasal septum, B- lateral wall of the nasal cavity).
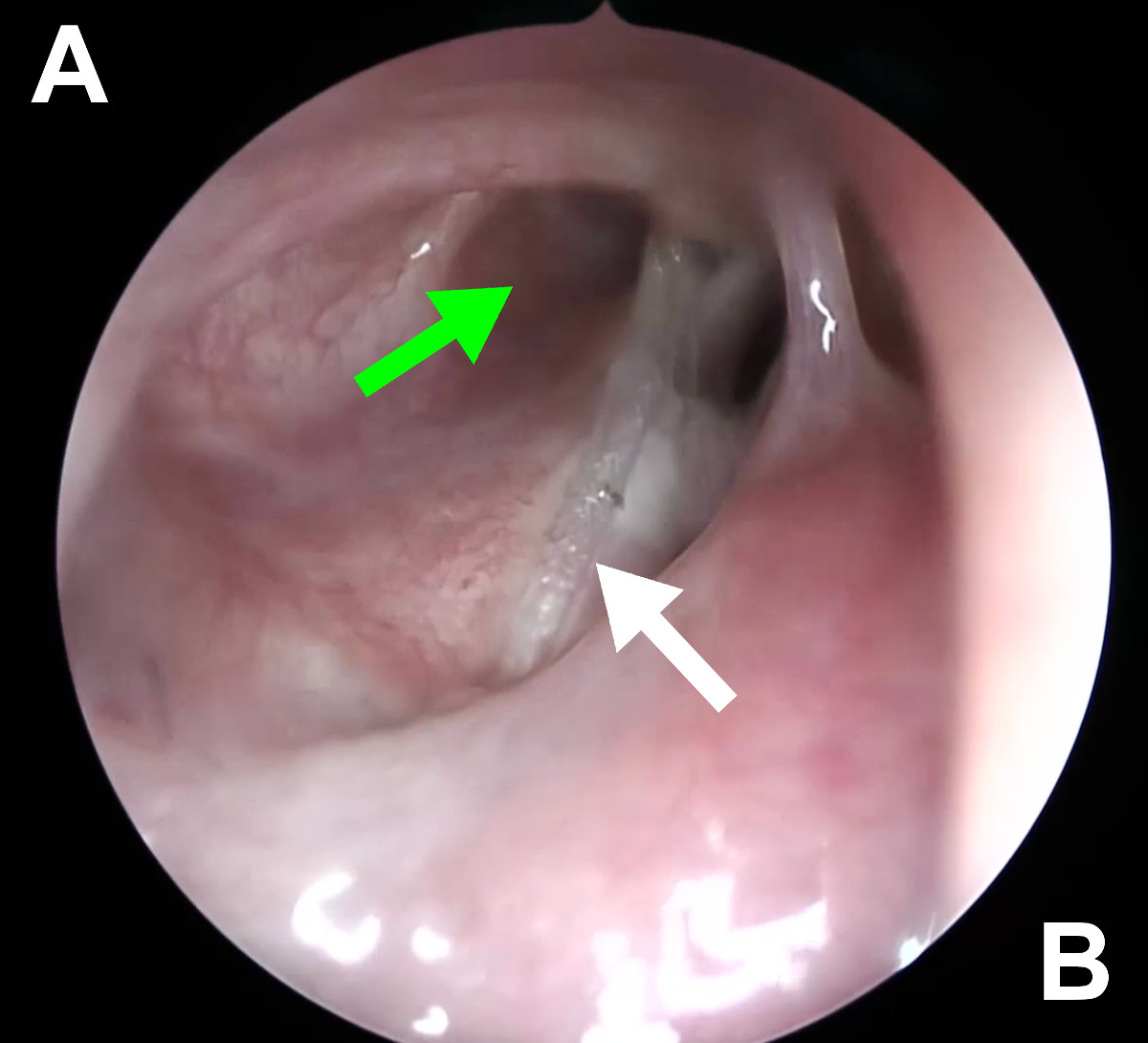 Figure 18. Endoscopic view of the right nasal cavity at 6 weeks postop
(Case 2): green arrow - patent ostium, white arrow – silicone stent.
Discussion
Numerous publications indicate that the effectiveness of endoscopic
dacryocystorhinostomy (EnDCR) is equal to or even surpasses the
effectiveness of external dacryocystorhinostomy (ExDCR)12, 13. This trend
results from the development of endoscopy, which allows
for better visualization of the surgical field inside the nasal cavity.
This is particularly important in atypical, traumatic cases of lacrimal
duct obstruction, where the facial anatomy may be significantly altered.
Such a situation occurs in the Le Fort fractures. In the past, the
endoscopic approach was not recommended for lacrimal duct obstruction
resulting from this type of fractures. The method of choice was ExDCR
due to what was considered the best surgical access14.
However, this approach did not allow for proper evaluation of the
surgeon's actions inside the nasal cavity, which is now possible with
the use of high-resolution endoscopes. Some authors have even suggested
performing dacryocystectomy (DCT) instead of DCR, which is currently
considered an inappropriate approach. In the cases described in this
publication, the surgeon was able to accurately assess the altered nasal
cavity anatomy and make appropriate decisions based on the evolving
situation. It allowed for the proper marsupialization of the lacrimal
sac under direct endoscopic control.
Ruya indicates that the use of 3D visualization with virtual reality
(VR) application can positively impact the surgeon's decision-making
during difficult surgeries15. In the three described cases,
surgical planning was performed based on conventional imaging and 3D
visualization, including VR. Such an approach could have influenced the
success of the procedures.
Ali proved that Mitomycin C circumostial injection improves the outcome
of EnDCR16. In the discussed cases, 0.02% Mitomycin C was
used in two applications: topically and in circumostial injections. This
might have had a part in preventing from ostium closure in the
postoperative period.
Conclusions
This study shows that endoscopic DCR can be conducted even in the most
complex posttraumatic obstructions of the lacrimal pathway. A step-wise
approach to such cases with a careful reading of the preoperative CT-DCG
and performing a complete marsupialization of the lacrimal sac gives an
excellent long-term outcome.
References
1. Ullrich K, Malhotra R, Patel BC. Dacryocystorhinostomy. [Updated
2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls
Publishing; 2023 Jan.
Figure 18. Endoscopic view of the right nasal cavity at 6 weeks postop
(Case 2): green arrow - patent ostium, white arrow – silicone stent.
Discussion
Numerous publications indicate that the effectiveness of endoscopic
dacryocystorhinostomy (EnDCR) is equal to or even surpasses the
effectiveness of external dacryocystorhinostomy (ExDCR)12, 13. This trend
results from the development of endoscopy, which allows
for better visualization of the surgical field inside the nasal cavity.
This is particularly important in atypical, traumatic cases of lacrimal
duct obstruction, where the facial anatomy may be significantly altered.
Such a situation occurs in the Le Fort fractures. In the past, the
endoscopic approach was not recommended for lacrimal duct obstruction
resulting from this type of fractures. The method of choice was ExDCR
due to what was considered the best surgical access14.
However, this approach did not allow for proper evaluation of the
surgeon's actions inside the nasal cavity, which is now possible with
the use of high-resolution endoscopes. Some authors have even suggested
performing dacryocystectomy (DCT) instead of DCR, which is currently
considered an inappropriate approach. In the cases described in this
publication, the surgeon was able to accurately assess the altered nasal
cavity anatomy and make appropriate decisions based on the evolving
situation. It allowed for the proper marsupialization of the lacrimal
sac under direct endoscopic control.
Ruya indicates that the use of 3D visualization with virtual reality
(VR) application can positively impact the surgeon's decision-making
during difficult surgeries15. In the three described cases,
surgical planning was performed based on conventional imaging and 3D
visualization, including VR. Such an approach could have influenced the
success of the procedures.
Ali proved that Mitomycin C circumostial injection improves the outcome
of EnDCR16. In the discussed cases, 0.02% Mitomycin C was
used in two applications: topically and in circumostial injections. This
might have had a part in preventing from ostium closure in the
postoperative period.
Conclusions
This study shows that endoscopic DCR can be conducted even in the most
complex posttraumatic obstructions of the lacrimal pathway. A step-wise
approach to such cases with a careful reading of the preoperative CT-DCG
and performing a complete marsupialization of the lacrimal sac gives an
excellent long-term outcome.
References
1. Ullrich K, Malhotra R, Patel BC. Dacryocystorhinostomy. [Updated
2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls
Publishing; 2023 Jan.
2. Perez Y, Patel BC, Mendez MD. Nasolacrimal Duct Obstruction. [Updated 2023 Jan 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
3. Nowak R, Rekas M, Gospodarowicz IN, Ali MJ. Long-term outcomes of primary transcanalicular laser dacryocystorhinostomy. Graefes Arch Clin Exp Ophthalmol. 2021 Aug;259(8):2425-2430. doi: 10.1007/s00417-021-05165-5. Epub 2021 Mar 26. PMID: 33770269.
4. Mukherjee B, Dhobekar M. Traumatic nasolacrimal duct obstruction: clinical profile, management, and outcome. Eur J Ophthalmol. 2013 Sep-Oct;23(5):615-22. doi: 10.5301/ejo.5000256. Epub 2013 Mar 20. PMID: 23516252.
5. Mukherjee B, Dhobekar M. Traumatic Nasolacrimal Duct Obstruction: Clinical Profile, Management, and Outcome. European Journal of Ophthalmology. 2013;23(5):615-622. doi:10.5301/ejo.5000256.
6. Keren S, Abergel A, Manor A, Rosenblatt A, Koenigstein D, Leibovitch I, Ben Cnaan R. Endoscopic dacryocystorhinostomy: reasons for failure. Eye (Lond). 2020 May;34(5):948-953. doi: 10.1038/s41433-019-0612-y. Epub 2019 Oct 8. PMID: 31595028; PMCID: PMC7182564.
7. Goodmaker C, Hohman MH, De Jesus O. Naso-Orbito-Ethmoid Fractures. [Updated 2023 Mar 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
8. Patel BC, Wright T, Waseem M. Le Fort Fractures. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
9. Wormald PJ. Powered endoscopic dacryocystorhinostomy. Laryngoscope. 2002 Jan;112(1):69-72. doi: 10.1097/00005537-200201000-00013. PMID: 11802041.
10. Ali MJ, Psaltis AJ, Murphy J, Wormald PJ. Powered endoscopic dacryocystorhinostomy: a decade of experience. Ophthalmic Plast Reconstr Surg. 2015 May-Jun;31(3):219-21. doi: 10.1097/IOP.0000000000000261. PMID: 25162414.
11. Nowak R, Ali MJ. Experience of the First Three Years of Independent Practice Following Surgical Training: Time Taken and Long-Term Outcomes of Powered Endoscopic Dacryocystorhinostomy. Semin Ophthalmol. 2023 Mar 29:1-5. doi: 10.1080/08820538.2023.2195025. Epub ahead of print. PMID: 36992526.
12. Jung SK, Kim YC, Cho WK, Paik JS, Yang SW. Surgical outcomes of endoscopic dacryocystorhinostomy: analysis of 1083 consecutive cases. Can J Ophthalmol. 2015 Dec;50(6):466-70. doi: 10.1016/j.jcjo.2015.08.007. PMID: 26651307.
13. Rizvi SA, Sharma SC, Tripathy S, Sharma S. Management of traumatic dacryocystitis and failed dacryocystorhinostomy using silicone lacrimal intubation set. Indian J Otolaryngol Head Neck Surg. 2011 Jul;63(3):264-8. doi: 10.1007/s12070-011-0230-x. Epub 2011 Apr 17. PMID: 22754807; PMCID: PMC3138957.
14. Ruyra X, Permanyer E, Huguet M, Maldonado G. Use of virtual reality for procedural planning of transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg. 2022 Oct 10;35(5):ivac248. doi: 10.1093/icvts/ivac248. PMID: 36205608; PMCID: PMC9639804.
15. Singh, Manpreet M.D.; Ali, Mohammad Javed F.R.C.S.; Naik, Milind N. M.D.. Long-Term Outcomes of Circumostial Injection of Mitomycin C (COS-MMC) in Dacryocystorhinostomy. Ophthalmic Plastic and Reconstructive Surgery 31(5):p 423-424, September/October 2015. | DOI: 10.1097/IOP.0000000000000491. For more information, contact info@medicalholodeck.com